Exploring Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
Exploring Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Exploring Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Exploring Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
- 2 Introduction
- 3 Exploring Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
- 3.1 Unveiling the Periodic Trends
- 3.2 Key Periodic Trends and Their Significance
- 3.3 Applications of Periodic Trends in Chemistry and Beyond
- 3.4 Periodic Trends Gizmo Answer Key 2025
- 3.4.1 Common Concepts Covered in the Gizmo:
- 3.4.2 Types of Questions You Might Encounter:
- 3.4.3 Tips for Using the Periodic Trends Gizmo:
- 3.5 Related Searches
- 3.6 FAQs
- 3.7 Conclusion
- 4 Closure
Exploring Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. Understanding these periodic trends is crucial for predicting chemical behavior and designing new materials. This article explores the key trends, their underlying causes, and their applications in various fields.
Unveiling the Periodic Trends
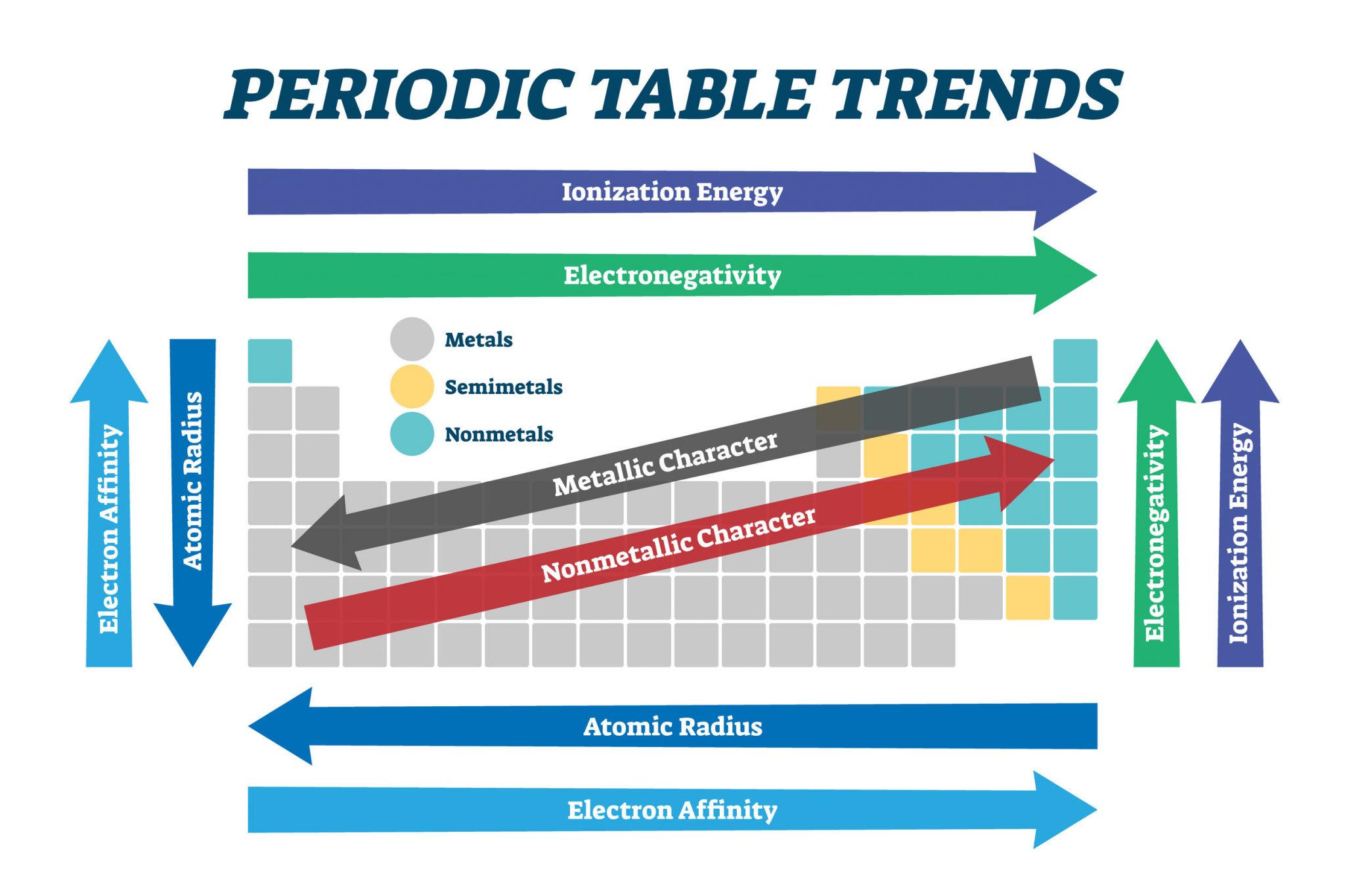
The periodic trends highlight the systematic variation in the properties of elements as we move across a period (row) or down a group (column) of the periodic table. These trends are primarily driven by two fundamental factors:
- Effective Nuclear Charge (Zeff): This refers to the net positive charge experienced by an electron in an atom. As we move across a period, Zeff increases due to the addition of protons in the nucleus without a corresponding increase in shielding electrons. This stronger attraction pulls electrons closer to the nucleus, leading to smaller atomic radii and higher ionization energies.
- Electron Shielding: Inner electrons shield outer electrons from the full attraction of the nucleus. As we move down a group, the number of electron shells increases, leading to increased shielding and a weaker attraction between the nucleus and outer electrons. This results in larger atomic radii and lower ionization energies.
Key Periodic Trends and Their Significance
-
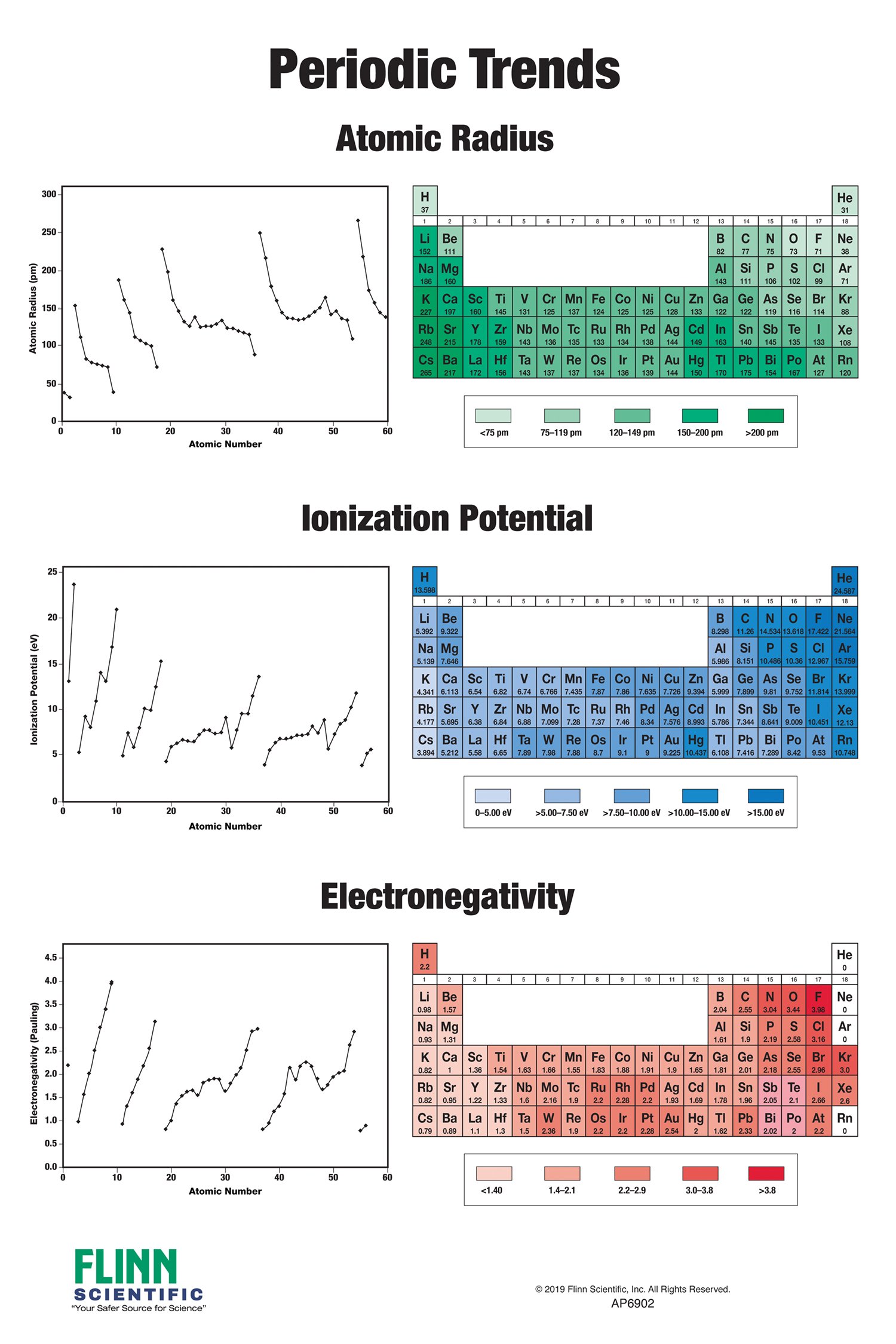
Atomic Radius: This trend refers to the distance between the nucleus and the outermost electron shell. Atomic radius decreases across a period due to increased Zeff, and it increases down a group due to increased electron shielding.
Applications: Understanding atomic radius is crucial for predicting the reactivity of elements, as smaller atoms tend to be more reactive. It also plays a role in determining the physical properties of materials, such as density and melting point.
-
Ionization Energy: The ionization energy is the minimum energy required to remove an electron from a gaseous atom in its ground state. Ionization energy generally increases across a period due to increased Zeff, and it decreases down a group due to increased electron shielding.
Applications: Ionization energy helps predict the ease with which an element forms cations (positively charged ions). Elements with high ionization energies are less likely to lose electrons, making them more stable.
-
Electron Affinity: Electron affinity is the change in energy when an electron is added to a neutral gaseous atom. It generally increases across a period due to increased Zeff, and it decreases down a group due to increased electron shielding. However, there are exceptions to this trend.
Applications: Electron affinity helps predict the ease with which an element forms anions (negatively charged ions). Elements with high electron affinities readily gain electrons, making them more stable.
-
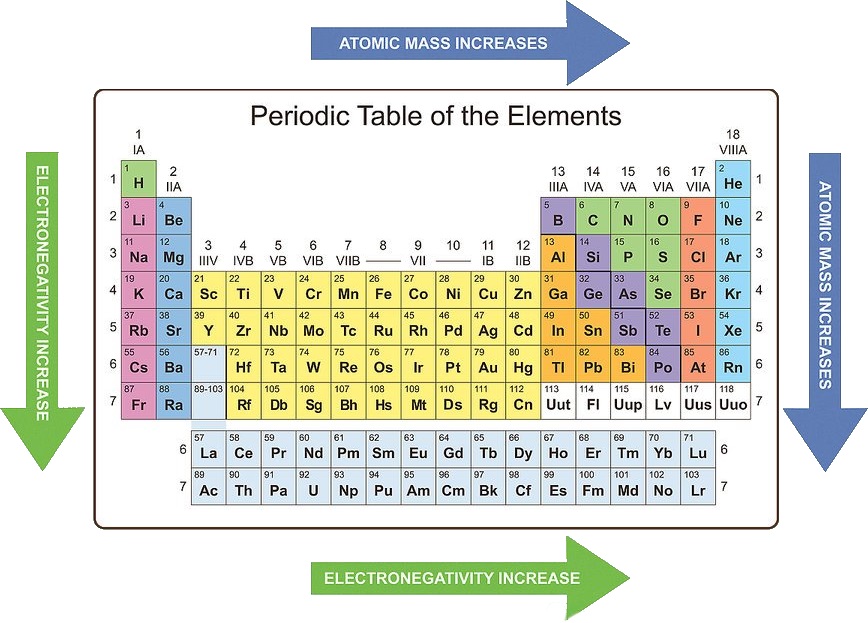
Electronegativity: Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. Electronegativity generally increases across a period due to increased Zeff, and it decreases down a group due to increased electron shielding.
Applications: Electronegativity is essential for understanding the nature of chemical bonds. It helps predict the polarity of a bond and the type of chemical reactions that an element is likely to participate in.
Applications of Periodic Trends in Chemistry and Beyond
Understanding periodic trends has numerous practical applications in various fields:
- Predicting Chemical Reactions: The trends in ionization energy, electron affinity, and electronegativity help predict the reactivity of elements and the products of chemical reactions.
- Designing New Materials: By manipulating the properties of elements through their position on the periodic table, scientists can design new materials with specific properties, such as semiconductors, catalysts, and superconductors.
- Understanding Biological Processes: Periodic trends influence the behavior of elements in biological systems. For example, the high electronegativity of oxygen explains its role in forming water and its importance in biological processes.
- Environmental Chemistry: Periodic trends are crucial for understanding the behavior of pollutants in the environment. For example, the high reactivity of halogens explains their role in ozone depletion.
Periodic Trends Gizmo Answer Key 2025
Note: The specific questions and answers in the Periodic Trends Gizmo may vary depending on the version and updates. However, the general principles and concepts remain consistent. This section will provide a general overview of the key concepts covered in the Gizmo and offer insights into common types of questions you might encounter.
Common Concepts Covered in the Gizmo:
- Atomic Radius: The Gizmo typically allows you to visualize and compare the atomic radii of different elements. You may be asked to identify trends in atomic radius across periods and down groups, explain the reasons behind these trends, and predict the relative sizes of atoms based on their position on the periodic table.
- Ionization Energy: The Gizmo often presents data on ionization energies and asks you to analyze the relationship between ionization energy and atomic structure. You might be asked to explain why ionization energy increases across a period and decreases down a group, predict the ionization energies of different elements, and apply this knowledge to understand the chemical behavior of elements.
- Electron Affinity: The Gizmo may explore electron affinity and its relationship to atomic structure. You could be asked to compare the electron affinities of different elements, explain the factors influencing electron affinity, and use this information to predict the formation of anions.
- Electronegativity: The Gizmo often includes information about electronegativity and its influence on chemical bonding. You might be asked to identify trends in electronegativity across the periodic table, explain the reasons behind these trends, and predict the polarity of bonds based on the electronegativity difference between atoms.
Types of Questions You Might Encounter:
- Multiple-Choice Questions: These questions test your understanding of key concepts related to periodic trends. For example, you might be asked to identify the element with the highest ionization energy or the element with the smallest atomic radius.
- Short Answer Questions: These questions require you to explain your reasoning and provide evidence to support your answers. For example, you might be asked to explain why atomic radius decreases across a period or why ionization energy increases across a period.
- Data Analysis: The Gizmo may provide data on atomic properties, such as atomic radius or ionization energy, and ask you to analyze and interpret this data. You might be asked to identify trends, draw conclusions, and make predictions based on the data.
Tips for Using the Periodic Trends Gizmo:
- Familiarize Yourself with the Gizmo Interface: Before starting the activity, spend some time exploring the Gizmo’s interface and understanding its features.
- Start with Basic Concepts: Begin by reviewing the key periodic trends and their underlying causes. This will provide a solid foundation for understanding the Gizmo’s activities.
- Experiment and Observe: The Gizmo allows you to manipulate variables and observe the effects on atomic properties. Use this feature to test your understanding and make connections between different concepts.
- Record Your Observations: Keep track of your observations and findings as you work through the Gizmo. This will help you to review the concepts later and identify areas where you need further clarification.
- Connect the Gizmo to Real-World Applications: Try to relate the concepts you learn in the Gizmo to real-world examples. This will help you to see how periodic trends are relevant to everyday life.
Related Searches
Understanding periodic trends is a fundamental aspect of chemistry, and many related searches can further enhance your knowledge:
- Periodic Table Trends: This search will lead you to resources that provide a comprehensive overview of the key periodic trends and their applications.
- Atomic Structure and Periodic Trends: This search will delve deeper into the relationship between atomic structure and the observed periodic trends.
- Periodic Trends Gizmo Activities: This search will help you find additional activities and resources related to the Periodic Trends Gizmo.
- Chemistry Textbook Periodic Trends: Searching for "periodic trends" in your chemistry textbook will provide a more in-depth explanation of the topic.
- Periodic Trends Worksheet Answers: This search can help you find practice problems and solutions related to periodic trends.
- Periodic Trends Quiz: This search will lead you to online quizzes and assessments that test your understanding of periodic trends.
- Periodic Trends and Chemical Bonding: This search will explore the connection between periodic trends and the formation of chemical bonds.
- Periodic Trends and Reactivity: This search will focus on the relationship between periodic trends and the reactivity of elements.
FAQs
Q: Why do atomic radii decrease across a period?
A: Atomic radii decrease across a period due to the increasing effective nuclear charge (Zeff). As we move across a period, the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and the electrons. This stronger attraction pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
Q: Why do ionization energies increase across a period?
A: Ionization energies increase across a period due to the increasing effective nuclear charge (Zeff). The stronger attraction between the nucleus and the electrons makes it more difficult to remove an electron, leading to a higher ionization energy.
Q: Why do electronegativities increase across a period?
A: Electronegativities increase across a period due to the increasing effective nuclear charge (Zeff). The stronger attraction between the nucleus and the electrons makes the atom more likely to attract electrons in a chemical bond, leading to a higher electronegativity.
Q: Why are noble gases so unreactive?
A: Noble gases are unreactive because they have a full outer shell of electrons, making them very stable. They have high ionization energies and low electron affinities, making it difficult for them to gain or lose electrons.
Q: How do periodic trends help us understand the reactivity of elements?
A: Periodic trends help us understand the reactivity of elements by providing insights into their tendency to gain or lose electrons. For example, elements with low ionization energies readily lose electrons, making them reactive metals. Elements with high electron affinities readily gain electrons, making them reactive nonmetals.
Conclusion
Understanding periodic trends is essential for comprehending the behavior of elements and predicting their chemical properties. The systematic variations in atomic radius, ionization energy, electron affinity, and electronegativity are driven by the interplay of effective nuclear charge and electron shielding. By applying these principles, we can unravel the mysteries of chemical reactions, design new materials, and gain insights into the fundamental processes that govern our world.

/periodictrendstable-5c4a46614cedfd000187c5db.jpg)
:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)


Closure
Thus, we hope this article has provided valuable insights into Exploring Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior. We appreciate your attention to our article. See you in our next article!
.PNG)