Understanding Atomic Radii Trends in the Periodic Table: A Look Ahead to 2025
Understanding Atomic Radii Trends in the Periodic Table: A Look Ahead to 2025
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Understanding Atomic Radii Trends in the Periodic Table: A Look Ahead to 2025. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Atomic Radii Trends in the Periodic Table: A Look Ahead to 2025
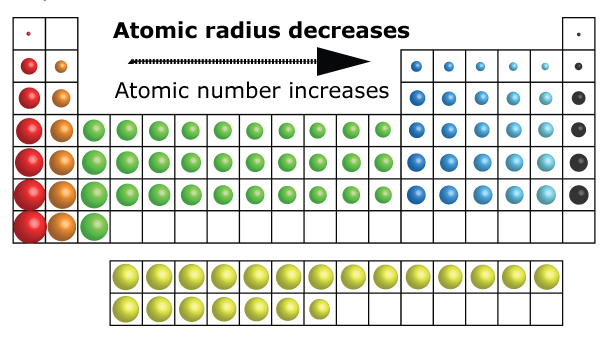
The periodic table, a cornerstone of chemistry, provides a systematic framework for understanding the properties of elements. One of the fundamental properties of atoms is their atomic radius, which is a measure of the size of an atom. Understanding the trends in atomic radius across the periodic table is crucial for predicting the behavior of elements and their compounds.
Atomic Radius Trends
The atomic radius of an element is influenced by two primary factors:
-
Nuclear Charge: As the number of protons in the nucleus increases, the electrostatic attraction between the nucleus and the electrons also increases, pulling the electrons closer to the nucleus and decreasing the atomic radius. This explains the trend of decreasing atomic radius across a period (from left to right).
-
Number of Electron Shells: As you move down a group (column) in the periodic table, the number of electron shells increases. This leads to an increase in the average distance between the nucleus and the outermost electrons, resulting in a larger atomic radius.
Predicting Atomic Radii in 2025
While the fundamental principles governing atomic radius trends remain constant, there are ongoing advancements in theoretical calculations and experimental techniques that allow for more precise predictions.
-
Computational Chemistry: Sophisticated quantum mechanical models are being employed to simulate the electronic structure of atoms and molecules. These models are constantly being refined, leading to more accurate estimations of atomic radii.
-
Experimental Techniques: Advanced spectroscopic methods, such as X-ray diffraction and electron scattering, are used to determine the size of atoms with increasing precision. These techniques are continually being improved, enabling more accurate measurements of atomic radii.
The Importance of Understanding Atomic Radius Trends
Knowledge of atomic radius trends is vital for various fields, including:
-
Chemistry: Understanding the size of atoms allows chemists to predict the reactivity of elements and their compounds. For example, smaller atoms with a higher nuclear charge tend to be more electronegative, attracting electrons more strongly.
-
Materials Science: The size of atoms plays a crucial role in determining the properties of materials. For example, the size of metal atoms influences their ductility and malleability.
-
Nanotechnology: Understanding atomic radii is essential in designing and synthesizing nanomaterials with specific properties. The size of atoms and their arrangement influence the properties of nanoparticles and other nanoscale structures.
Related Searches
Here are some related searches that delve deeper into the concepts surrounding atomic radii:
-
Atomic Radius Trends in Groups: Explore the systematic increase in atomic radius as you move down a group in the periodic table.
-
Atomic Radius Trends in Periods: Understand the gradual decrease in atomic radius across a period due to increasing nuclear charge.
-
Ionic Radius Trends: Investigate how the formation of ions influences the atomic radius, leading to variations in ionic size.
-
Covalent Radius Trends: Delve into the concept of covalent radius, which measures the distance between the nuclei of two covalently bonded atoms.
-
Metallic Radius Trends: Explore the trends in metallic radius, which is defined as half the distance between the nuclei of two adjacent metal atoms.
-
Van der Waals Radius Trends: Understand the concept of Van der Waals radius, which represents the distance between the nuclei of two non-bonded atoms.
-
Atomic Radius and Electronegativity: Investigate the relationship between atomic radius and electronegativity, where smaller atoms tend to be more electronegative.
-
Atomic Radius and Ionization Energy: Explore the correlation between atomic radius and ionization energy, where smaller atoms require more energy to remove an electron.
FAQs
Q: How does the atomic radius change with the number of protons?
A: As the number of protons in the nucleus increases (moving across a period), the electrostatic attraction between the nucleus and electrons strengthens, pulling the electrons closer and decreasing the atomic radius.
Q: Why does the atomic radius increase down a group?
A: Moving down a group, the number of electron shells increases, leading to a larger distance between the nucleus and the outermost electrons, resulting in a larger atomic radius.
Q: How do ionic radii compare to atomic radii?
A: Cations (positively charged ions) are smaller than their corresponding neutral atoms due to the loss of electrons. Conversely, anions (negatively charged ions) are larger than their corresponding neutral atoms due to the gain of electrons.
Q: What are the applications of atomic radius trends in materials science?
A: Atomic radius plays a crucial role in determining the properties of materials. For example, the size of metal atoms influences their ductility and malleability. Smaller atoms can pack more tightly, leading to stronger metallic bonds and increased hardness.
Q: How are atomic radius trends used in nanotechnology?
A: Understanding atomic radii is essential in designing and synthesizing nanomaterials with specific properties. The size of atoms and their arrangement influence the properties of nanoparticles and other nanoscale structures.
Tips for Understanding Atomic Radius Trends
- Visualize the Periodic Table: Use a periodic table with atomic radius values to observe the trends visually.
- Focus on the Fundamental Factors: Remember that nuclear charge and the number of electron shells are the primary drivers of atomic radius trends.
- Consider the Formation of Ions: Understand how the gain or loss of electrons affects the size of an atom, resulting in ionic radii.
- Relate Atomic Radius to Other Properties: Explore the connections between atomic radius and other properties like electronegativity, ionization energy, and reactivity.
Conclusion
The trends in atomic radius across the periodic table provide a fundamental framework for understanding the behavior of elements and their compounds. By recognizing the influence of nuclear charge and the number of electron shells, we can predict and explain the size of atoms. As computational models and experimental techniques continue to advance, our understanding of atomic radius trends will become even more precise, paving the way for further breakthroughs in various fields, including chemistry, materials science, and nanotechnology.

/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)


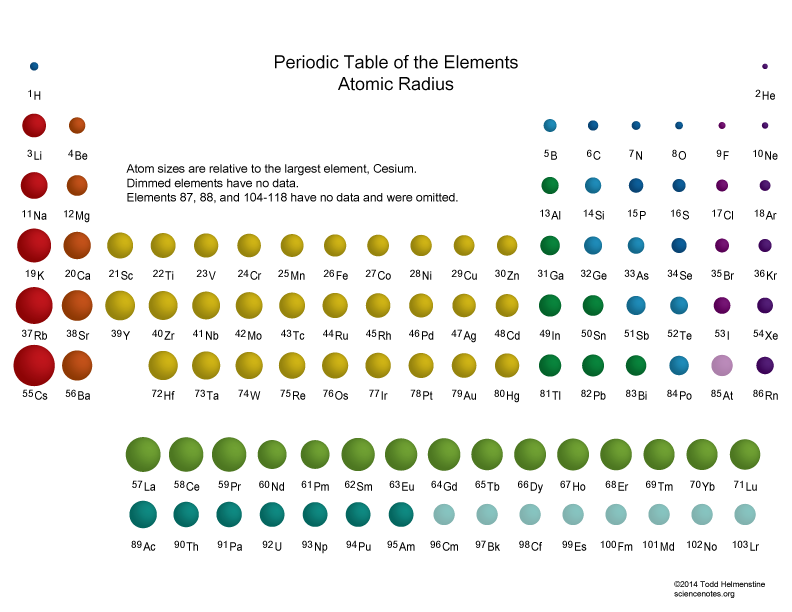
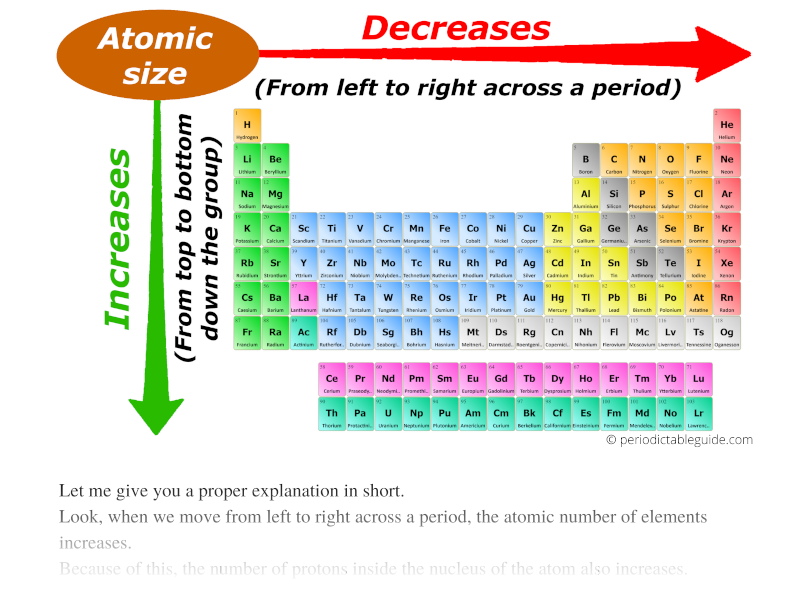
Closure
Thus, we hope this article has provided valuable insights into Understanding Atomic Radii Trends in the Periodic Table: A Look Ahead to 2025. We thank you for taking the time to read this article. See you in our next article!