Understanding the Periodic Trends in Reactivity: A 2025 Perspective
Understanding the Periodic Trends in Reactivity: A 2025 Perspective
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding the Periodic Trends in Reactivity: A 2025 Perspective. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Understanding the Periodic Trends in Reactivity: A 2025 Perspective
- 2 Introduction
- 3 Understanding the Periodic Trends in Reactivity: A 2025 Perspective
- 3.1 Trends in Reactivity Across the Periodic Table
- 3.2 Understanding the Importance of Periodic Trends in Reactivity
- 3.3 Related Searches
- 3.4 FAQs about Periodic Trends in Reactivity
- 3.5 Tips for Understanding and Applying Periodic Trends in Reactivity
- 3.6 Conclusion
- 4 Closure
Understanding the Periodic Trends in Reactivity: A 2025 Perspective

The periodic table is a fundamental tool in chemistry, providing a systematic organization of elements based on their properties. One of the most important and impactful trends within this organization is the periodic trend in reactivity. This trend governs how readily elements participate in chemical reactions, influencing the formation of compounds and impacting a wide range of scientific and technological fields.
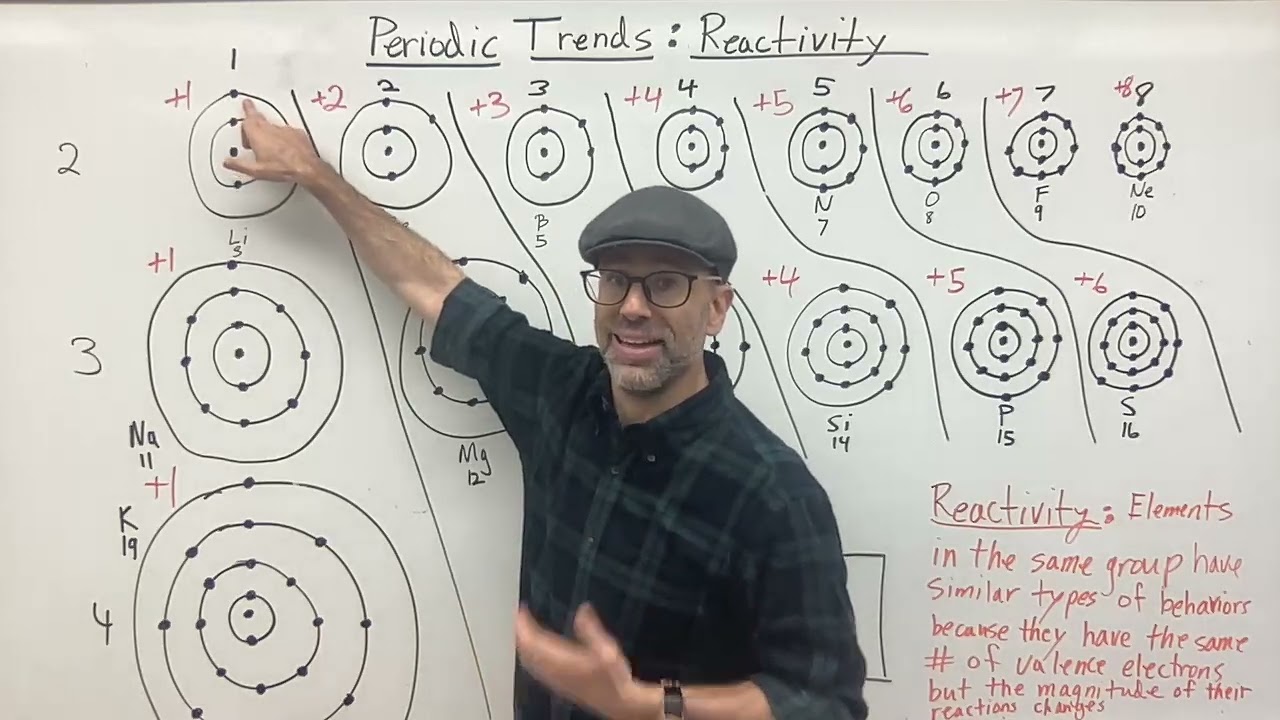
Periodic trends in reactivity are governed by the fundamental properties of atoms: their electron configuration, ionization energy, electronegativity, and electron affinity. These properties, in turn, are influenced by the position of an element within the periodic table, specifically its period (horizontal row) and group (vertical column).
Understanding these trends is crucial for predicting chemical behavior, designing new materials, and developing innovative technologies.
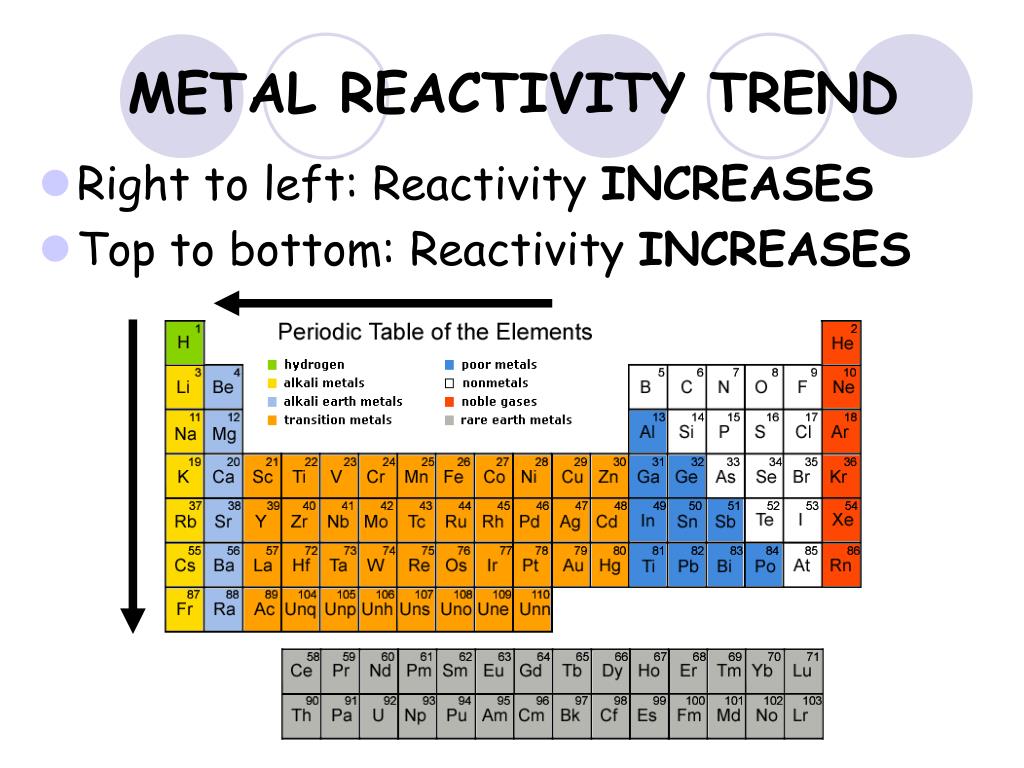
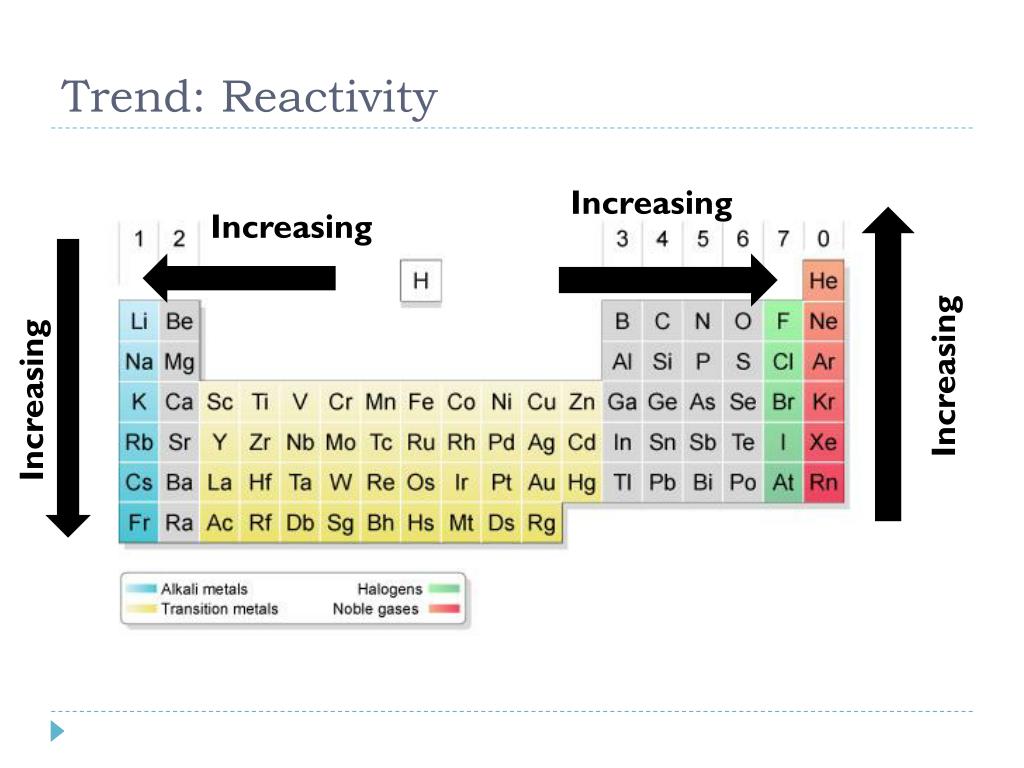
Trends in Reactivity Across the Periodic Table
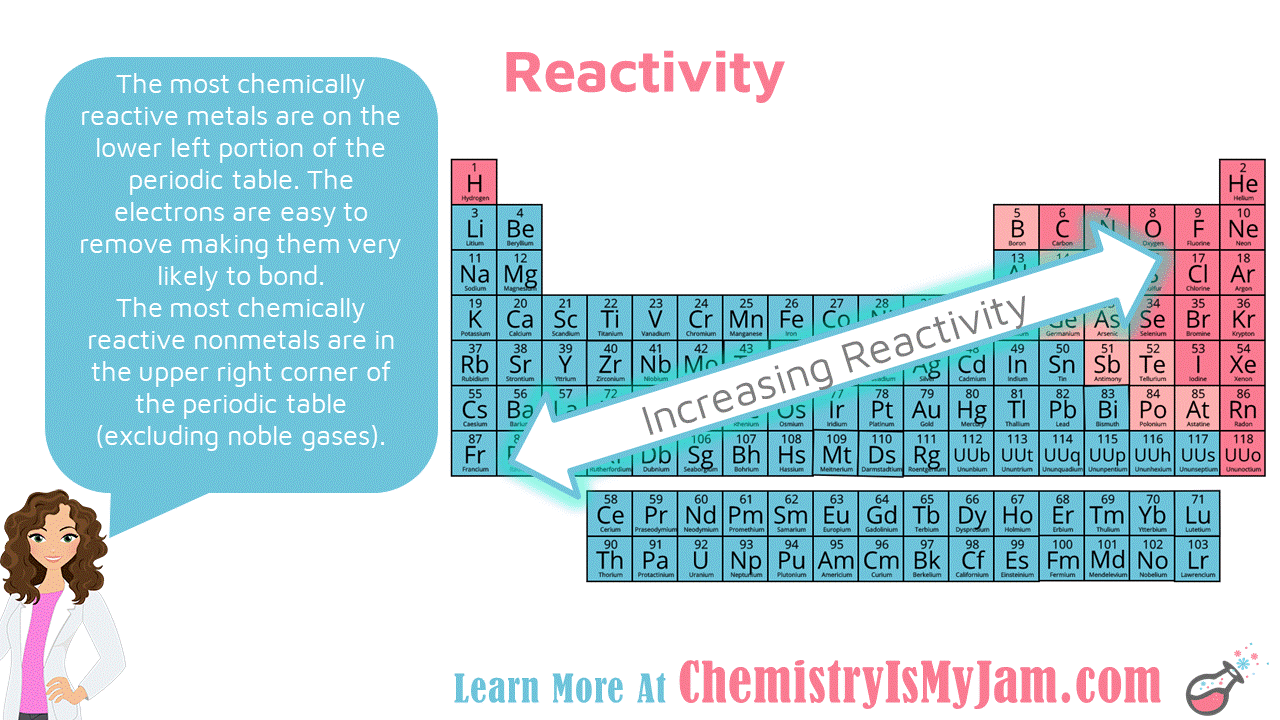
1. Reactivity of Metals:
Metals, located on the left side of the periodic table, generally exhibit high reactivity. This is due to their tendency to lose electrons and form positive ions (cations).
- Across a period: As you move from left to right across a period, the metallic character decreases, and reactivity diminishes. This is because the atomic radius decreases, making it harder for the outermost electrons to be lost.
- Down a group: Moving down a group, metallic character and reactivity increase. This is because the outermost electron is further from the nucleus and experiences less attraction, making it easier to lose.
Examples:
- Alkali Metals (Group 1): Highly reactive due to their single valence electron, readily reacting with water and air.
- Alkaline Earth Metals (Group 2): Less reactive than alkali metals due to their two valence electrons, but still reactive enough to react with water and oxygen.
- Transition Metals: Exhibit a wide range of reactivity depending on their electron configuration and oxidation states.
2. Reactivity of Nonmetals:
Nonmetals, situated on the right side of the periodic table, generally exhibit high electronegativity and a tendency to gain electrons to form negative ions (anions).
- Across a period: Nonmetallic character increases as you move across a period, leading to higher reactivity. The smaller atomic radius allows for stronger attraction to incoming electrons.
- Down a group: Nonmetallic character and reactivity decrease as you move down a group. The larger atomic radius weakens the attraction between the nucleus and incoming electrons.
Examples:
- Halogens (Group 17): Highly reactive nonmetals due to their high electronegativity and tendency to gain one electron to achieve a stable noble gas configuration.
- Oxygen (Group 16): A highly reactive nonmetal, readily forming oxides with many elements.
- Nitrogen (Group 15): Less reactive than oxygen but still forms important compounds, like nitrogen oxides and ammonia.
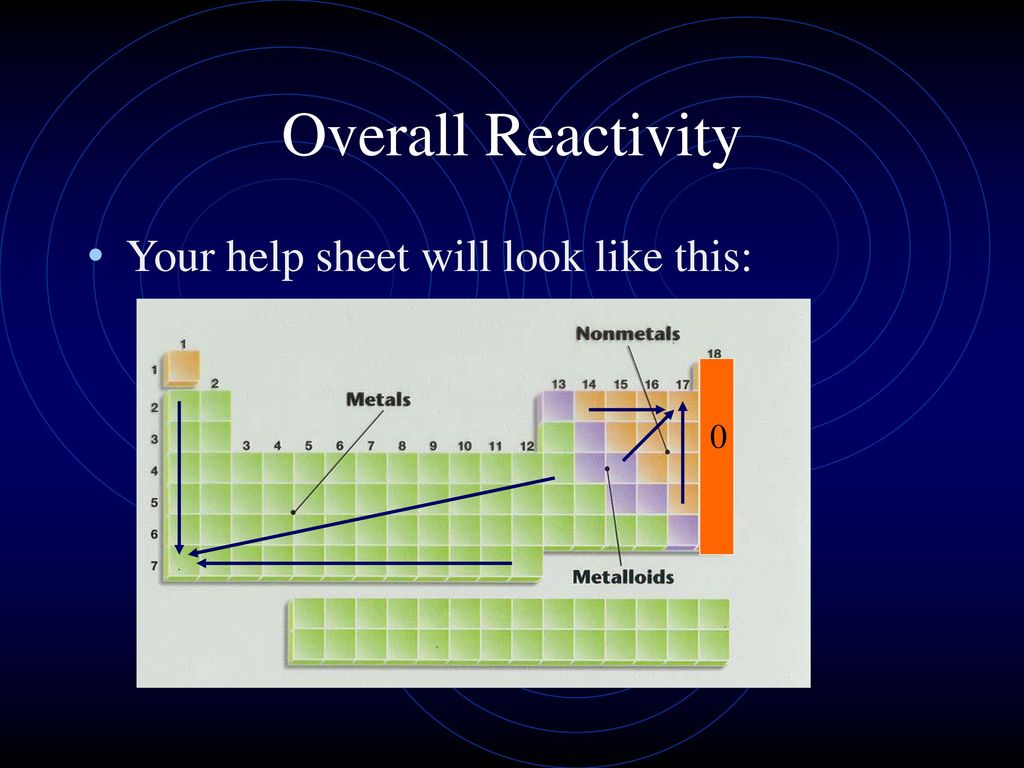
3. Reactivity of Metalloids:
Metalloids, situated along the "staircase" line separating metals and nonmetals, exhibit properties of both metals and nonmetals. Their reactivity is often intermediate and can be influenced by factors like temperature and the presence of other elements.
Examples:
- Silicon (Si): A key component of semiconductors and solar panels, silicon’s reactivity is influenced by temperature and the presence of other elements.
- Germanium (Ge): Used in transistors and infrared detectors, germanium’s reactivity is also influenced by temperature and other elements.
Understanding the Importance of Periodic Trends in Reactivity
1. Predicting Chemical Reactions:
The periodic trends in reactivity are essential for predicting the likelihood and outcome of chemical reactions. Understanding the relative reactivity of elements allows chemists to predict which elements will readily react with each other and what products will be formed.
2. Designing New Materials:
The reactivity of elements plays a crucial role in materials science. By understanding the trends, researchers can design materials with specific properties, such as strength, conductivity, and heat resistance. For example, understanding the reactivity of transition metals allows for the development of alloys with specific properties for applications like aerospace, construction, and automotive industries.
3. Understanding Biological Processes:
The reactivity of elements is essential for understanding biological processes. The reactivity of elements like carbon, hydrogen, oxygen, and nitrogen drives the formation of organic molecules that are fundamental to life. Understanding the reactivity of these elements helps us comprehend the mechanisms of biological processes like photosynthesis, respiration, and enzyme activity.
4. Developing New Technologies:
The understanding of periodic trends in reactivity is crucial for developing new technologies. For example, the reactivity of lithium and cobalt is essential for the development of lithium-ion batteries, a key technology for electric vehicles and renewable energy storage.
Related Searches
The periodic trends in reactivity are a broad topic with numerous related areas of exploration. Here are some related searches that provide deeper insights into different aspects of this concept:
1. Reactivity Series:
A list of metals arranged in order of their decreasing reactivity, which helps predict the outcome of displacement reactions.
2. Electronegativity Trends:
The tendency of an atom to attract electrons in a chemical bond, which influences the type of bond formed and the polarity of molecules.
3. Ionization Energy Trends:
The energy required to remove an electron from an atom, which influences the ease with which an element forms cations.
4. Electron Affinity Trends:
The energy change associated with the addition of an electron to an atom, which influences the ease with which an element forms anions.
5. Chemical Bonding and Reactivity:
The relationship between the type of chemical bond formed and the reactivity of elements.
6. Redox Reactions and Reactivity:
The role of oxidation and reduction reactions in determining the reactivity of elements.
7. Applications of Periodic Trends in Reactivity:
Examples of how the understanding of periodic trends in reactivity has led to the development of new materials and technologies.
8. Historical Development of Periodic Trends in Reactivity:
The evolution of our understanding of the periodic trends in reactivity from early observations to modern theories.
FAQs about Periodic Trends in Reactivity
1. Why do elements in the same group have similar reactivity?
Elements in the same group have the same number of valence electrons, which are the outermost electrons involved in chemical bonding. This similarity in electron configuration leads to similar reactivity patterns.
2. How does electronegativity affect reactivity?
Elements with high electronegativity tend to attract electrons from other atoms, making them more likely to gain electrons and form anions. This higher electronegativity generally leads to higher reactivity in nonmetals.
3. What is the relationship between ionization energy and reactivity?
Elements with low ionization energy readily lose electrons, making them more reactive as metals. Conversely, elements with high ionization energy require more energy to lose electrons, making them less reactive.
4. How can I predict the reactivity of an element based on its position in the periodic table?
By considering the element’s position in the periodic table, specifically its period and group, you can predict its reactivity. Elements in the same period generally exhibit similar reactivity trends, while elements in the same group share similar reactivity patterns.
5. What are some real-world applications of understanding periodic trends in reactivity?
Understanding periodic trends in reactivity has numerous applications in various fields. For example, it helps in designing new materials with specific properties, developing efficient batteries, and understanding biological processes.
6. Can the reactivity of an element change based on its environment?
Yes, the reactivity of an element can be influenced by its environment. For example, the presence of other elements, temperature, and pressure can affect the reactivity of an element.
7. What are some limitations of using periodic trends to predict reactivity?
While periodic trends in reactivity provide a valuable framework for predicting chemical behavior, they are not always perfect predictors. Factors like the specific reaction conditions, the presence of catalysts, and the formation of complex ions can influence the outcome of reactions and deviate from expected trends.
Tips for Understanding and Applying Periodic Trends in Reactivity
1. Visualize the Periodic Table:
Use a periodic table as a visual aid to understand the trends in reactivity. The position of an element within the table provides valuable information about its properties.
2. Focus on Valence Electrons:
Pay close attention to the number of valence electrons an element has, as this directly influences its reactivity.
3. Consider Electronegativity:
Electronegativity is a key factor in determining the reactivity of an element, especially for nonmetals.
4. Practice with Examples:
Work through examples of different chemical reactions to see how the reactivity of elements influences the outcome.
5. Explore Real-World Applications:
Learn about the practical applications of understanding periodic trends in reactivity in various fields, such as materials science, medicine, and technology.
Conclusion
Periodic trends in reactivity are fundamental concepts in chemistry, providing a framework for understanding and predicting the behavior of elements. By understanding these trends, we can gain valuable insights into the formation of compounds, the design of new materials, and the development of innovative technologies.
The periodic table, with its organized structure and predictable trends, remains a powerful tool for understanding the fundamental properties of elements and their interactions, ultimately contributing to advancements in various scientific and technological fields.


:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Closure
Thus, we hope this article has provided valuable insights into Understanding the Periodic Trends in Reactivity: A 2025 Perspective. We appreciate your attention to our article. See you in our next article!