Understanding the Periodic Trends of Ionic Radius: A Comprehensive Guide
Understanding the Periodic Trends of Ionic Radius: A Comprehensive Guide
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Understanding the Periodic Trends of Ionic Radius: A Comprehensive Guide. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Understanding the Periodic Trends of Ionic Radius: A Comprehensive Guide
- 2 Introduction
- 3 Understanding the Periodic Trends of Ionic Radius: A Comprehensive Guide
- 3.1 What is Ionic Radius?
- 3.2 Periodic Trends of Ionic Radius
- 3.3 Factors Affecting Ionic Radius
- 3.4 Importance of Understanding Ionic Radius
- 3.5 Related Searches
- 3.5.1 1. Ionic Radius Trends in the Periodic Table
- 3.5.2 2. Factors Affecting Ionic Radius
- 3.5.3 3. Ionic Radius and Chemical Reactivity
- 3.5.4 4. Ionic Radius and Physical Properties
- 3.5.5 5. Ionic Radius and Crystal Structure
- 3.5.6 6. Ionic Radius and Chemical Reactions
- 3.5.7 7. Ionic Radius and Bonding
- 3.5.8 8. Applications of Ionic Radius
- 3.6 FAQs
- 3.6.9 1. What is the difference between ionic radius and atomic radius?
- 3.6.10 2. How do you determine the ionic radius of an element?
- 3.6.11 3. Why is ionic radius important in chemistry?
- 3.6.12 4. What are some examples of how ionic radius affects chemical reactivity?
- 3.6.13 5. How does ionic radius relate to the solubility of ionic compounds?
- 3.7 Tips
- 3.8 Conclusion
- 4 Closure
Understanding the Periodic Trends of Ionic Radius: A Comprehensive Guide

The periodic table is a powerful tool in chemistry, organizing elements based on their properties and revealing predictable patterns. One such pattern is the periodic trend of ionic radius, which describes the size of an ion relative to its parent atom. This trend plays a crucial role in understanding the behavior of elements, particularly their chemical reactivity and the formation of ionic compounds.
What is Ionic Radius?
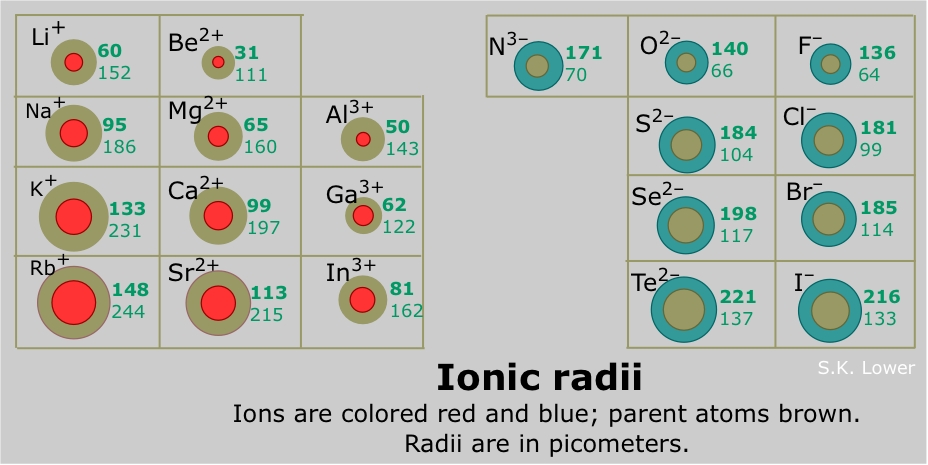
Ionic radius refers to the distance between the nucleus of an ion and its outermost electron shell. It is a measure of the size of an ion, which is different from the atomic radius of the neutral atom. When an atom gains or loses electrons to form an ion, its size changes.
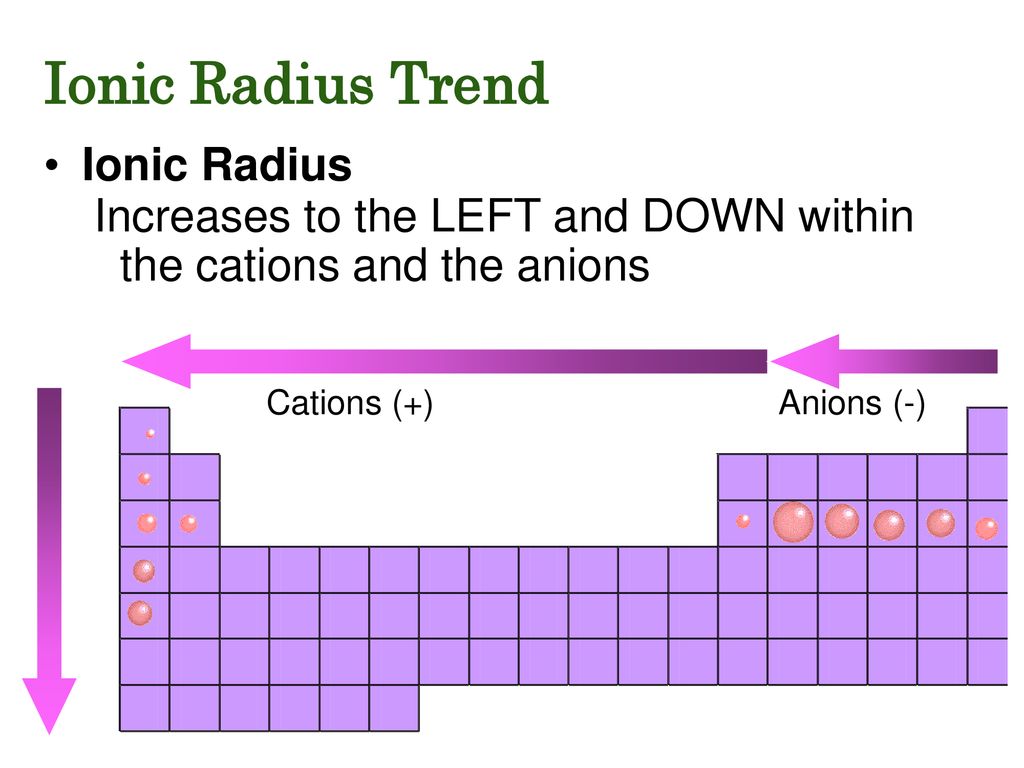
- Cations: Positively charged ions (formed by losing electrons) are smaller than their parent atoms. This is because the loss of electrons reduces electron-electron repulsion, allowing the remaining electrons to be pulled closer to the nucleus.
- Anions: Negatively charged ions (formed by gaining electrons) are larger than their parent atoms. This is because the addition of electrons increases electron-electron repulsion, pushing the electrons further away from the nucleus.
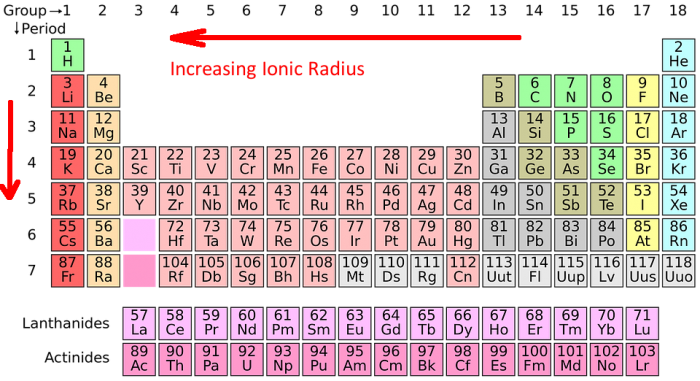
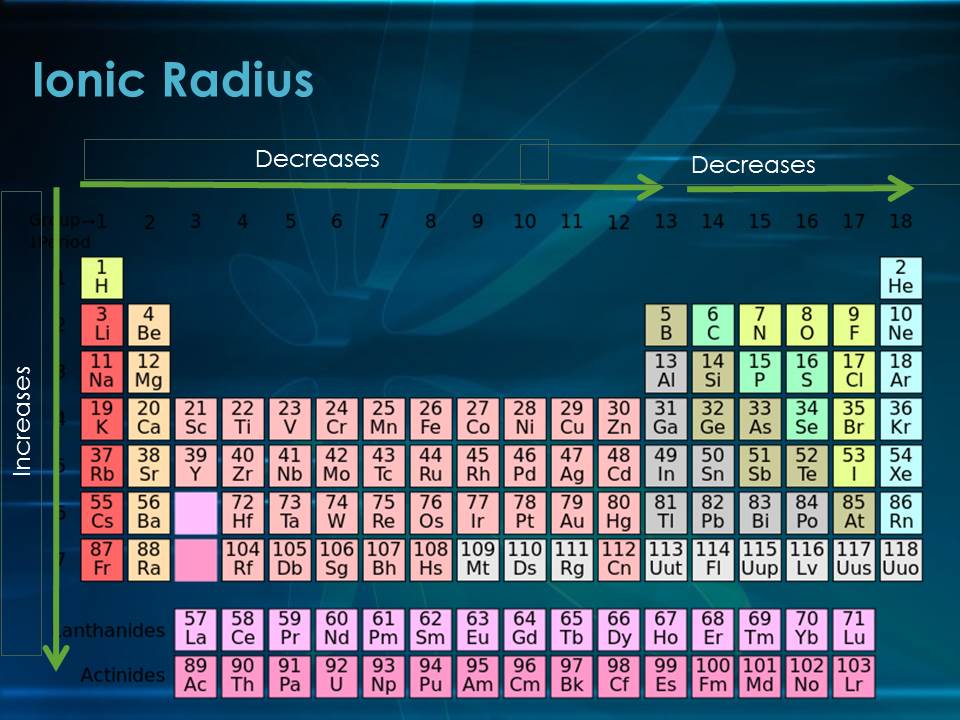
Periodic Trends of Ionic Radius
The periodic trends of ionic radius are as follows:
-
Across a Period (Left to Right): Ionic radius decreases as you move across a period from left to right. This is because the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and the electrons. This attraction pulls the electrons closer to the nucleus, resulting in a smaller ionic radius.
-
Down a Group (Top to Bottom): Ionic radius increases as you move down a group from top to bottom. This is because the number of electron shells increases. As you move down a group, each element adds a new electron shell, pushing the outermost electrons further away from the nucleus. This increased distance results in a larger ionic radius.
Factors Affecting Ionic Radius
Several factors contribute to the variation in ionic radius:
- Nuclear Charge: A higher nuclear charge (more protons) leads to a stronger attraction between the nucleus and electrons, resulting in a smaller ionic radius.
- Number of Electron Shells: As the number of electron shells increases, the electrons are further from the nucleus, leading to a larger ionic radius.
- Electron-Electron Repulsion: Increased electron-electron repulsion in anions pushes the electrons further from the nucleus, resulting in a larger ionic radius.
- Effective Nuclear Charge: The effective nuclear charge experienced by an electron is the net positive charge that it feels. It is determined by the nuclear charge and the shielding effect of inner electrons. A higher effective nuclear charge leads to a smaller ionic radius.
Importance of Understanding Ionic Radius
Understanding periodic trends of ionic radius is crucial for several reasons:
- Predicting Chemical Reactivity: The size of an ion plays a significant role in its chemical reactivity. Smaller ions are more likely to form ionic bonds with larger ions, leading to the formation of stable ionic compounds.
- Explaining Physical Properties: The ionic radius influences the physical properties of ionic compounds, such as melting point, boiling point, and solubility.
- Understanding Crystal Structure: The size of ions determines the arrangement of ions in a crystal lattice, impacting the overall structure and properties of the solid.
- Predicting Chemical Reactions: Knowledge of ionic radius helps predict the outcome of chemical reactions involving ions.
Related Searches
1. Ionic Radius Trends in the Periodic Table
Understanding the trends of ionic radius across periods and groups is fundamental to predicting the size and behavior of ions. The decrease in ionic radius across a period is due to the increasing nuclear charge pulling electrons closer, while the increase down a group is caused by the addition of new electron shells.
2. Factors Affecting Ionic Radius
Several factors contribute to the variation in ionic radius, including nuclear charge, number of electron shells, electron-electron repulsion, and effective nuclear charge. Each factor influences the strength of attraction between the nucleus and electrons, ultimately determining the size of the ion.
3. Ionic Radius and Chemical Reactivity
The size of an ion plays a significant role in its chemical reactivity. Smaller ions are more likely to form ionic bonds with larger ions, leading to the formation of stable ionic compounds. This is due to the electrostatic attraction between oppositely charged ions, which is stronger when the ions are smaller.
4. Ionic Radius and Physical Properties
Ionic radius influences the physical properties of ionic compounds, such as melting point, boiling point, and solubility. Larger ions tend to have lower melting and boiling points due to weaker electrostatic attractions. Similarly, larger ions are more likely to be soluble in water as they can interact more effectively with water molecules.
5. Ionic Radius and Crystal Structure
The size of ions determines the arrangement of ions in a crystal lattice. Larger ions require more space, leading to different crystal structures. Understanding the relationship between ionic radius and crystal structure is crucial for predicting the properties of solid ionic compounds.
6. Ionic Radius and Chemical Reactions
Knowledge of ionic radius helps predict the outcome of chemical reactions involving ions. For instance, the reaction between a small cation and a large anion is more likely to occur than the reaction between a large cation and a small anion.
7. Ionic Radius and Bonding
Ionic radius plays a crucial role in determining the type of bond formed between atoms. Smaller ions are more likely to form ionic bonds, while larger ions are more likely to form covalent bonds.
8. Applications of Ionic Radius
Understanding ionic radius has numerous applications in various fields, including:
- Materials Science: Predicting the properties of new materials based on their ionic composition.
- Biochemistry: Understanding the interactions between ions and biological molecules.
- Environmental Chemistry: Predicting the fate and transport of ions in the environment.
FAQs
1. What is the difference between ionic radius and atomic radius?
Ionic radius refers to the size of an ion, which is different from the atomic radius of the neutral atom. When an atom gains or loses electrons to form an ion, its size changes. Cations are smaller than their parent atoms, while anions are larger.
2. How do you determine the ionic radius of an element?
Ionic radius is usually determined experimentally using X-ray diffraction techniques. These techniques allow scientists to measure the distances between ions in a crystal lattice, providing information about their ionic radii.
3. Why is ionic radius important in chemistry?
Understanding ionic radius is crucial for predicting the chemical reactivity, physical properties, crystal structure, and chemical reactions of ions. It helps scientists understand the behavior of elements and design new materials with specific properties.
4. What are some examples of how ionic radius affects chemical reactivity?
Smaller ions are more likely to form ionic bonds with larger ions, leading to the formation of stable ionic compounds. For example, lithium (Li+) is a small cation and forms stable ionic compounds with large anions like chloride (Cl-) and bromide (Br-).
5. How does ionic radius relate to the solubility of ionic compounds?
Larger ions tend to be more soluble in water as they can interact more effectively with water molecules. This is because the larger surface area of the ion provides more opportunities for hydrogen bonding with water molecules.
Tips
- Use the periodic table: The periodic table provides a visual representation of the trends of ionic radius, making it easier to predict the size of ions.
- Consider the charge of the ion: Remember that cations are smaller than their parent atoms, while anions are larger.
- Focus on the factors affecting ionic radius: Understand the role of nuclear charge, number of electron shells, electron-electron repulsion, and effective nuclear charge in determining ionic radius.
- Practice with examples: Work through examples to solidify your understanding of the periodic trends of ionic radius.
Conclusion
Understanding the periodic trends of ionic radius is essential for comprehending the behavior of elements and predicting their chemical reactivity, physical properties, crystal structure, and chemical reactions. By recognizing the factors that influence ionic radius and applying the periodic trends, scientists can gain valuable insights into the properties of ionic compounds and design new materials with specific characteristics.


:max_bytes(150000):strip_icc()/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
Closure
Thus, we hope this article has provided valuable insights into Understanding the Periodic Trends of Ionic Radius: A Comprehensive Guide. We hope you find this article informative and beneficial. See you in our next article!