Understanding the Trends of Electron Affinity in the Periodic Table
Understanding the Trends of Electron Affinity in the Periodic Table
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding the Trends of Electron Affinity in the Periodic Table. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding the Trends of Electron Affinity in the Periodic Table

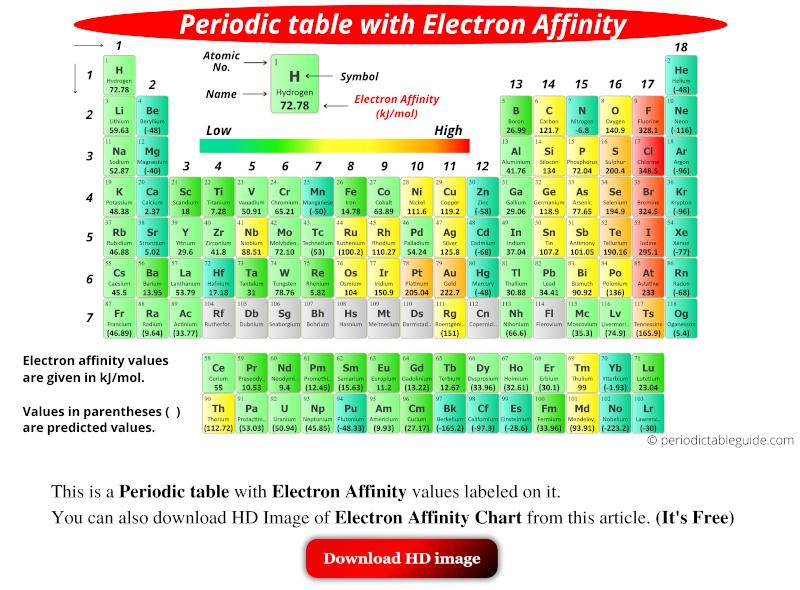
The periodic table, a fundamental tool in chemistry, organizes elements based on their recurring properties. One such property is electron affinity, the change in energy when an atom gains an electron in its gaseous state. Understanding the trends of electron affinity within the periodic table is crucial for predicting chemical reactivity and understanding the formation of chemical bonds.
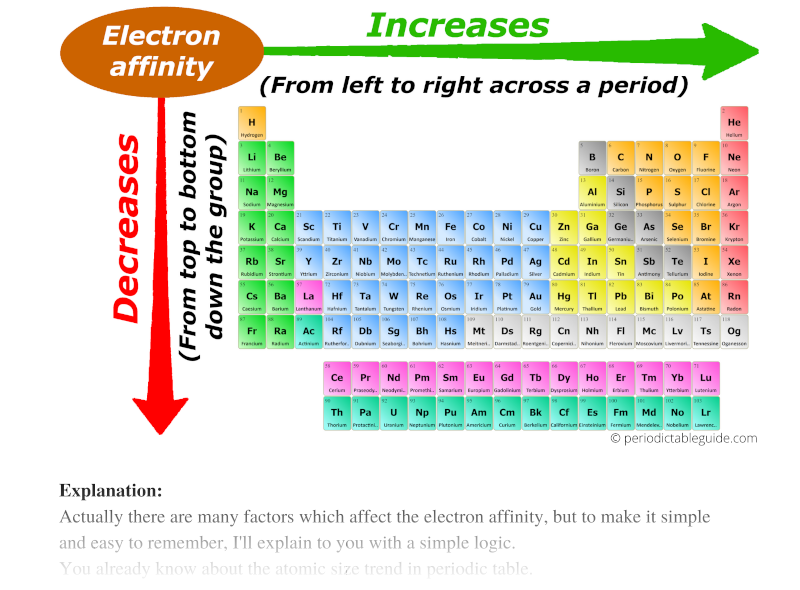
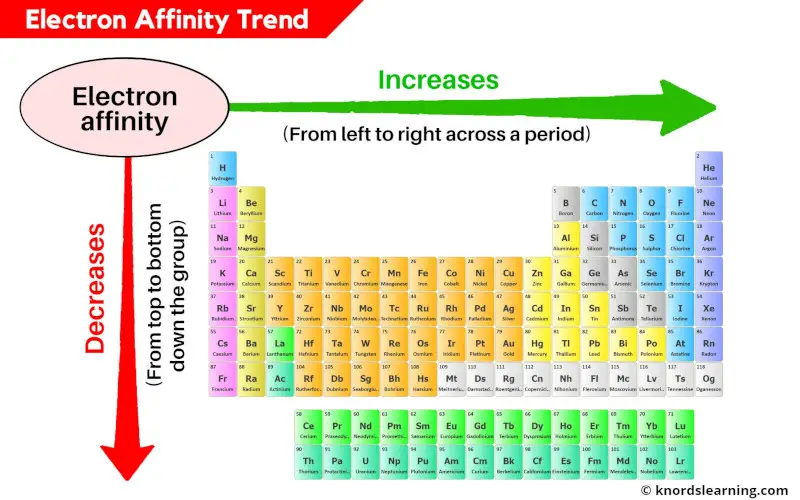
Trends of Electron Affinity in the Periodic Table
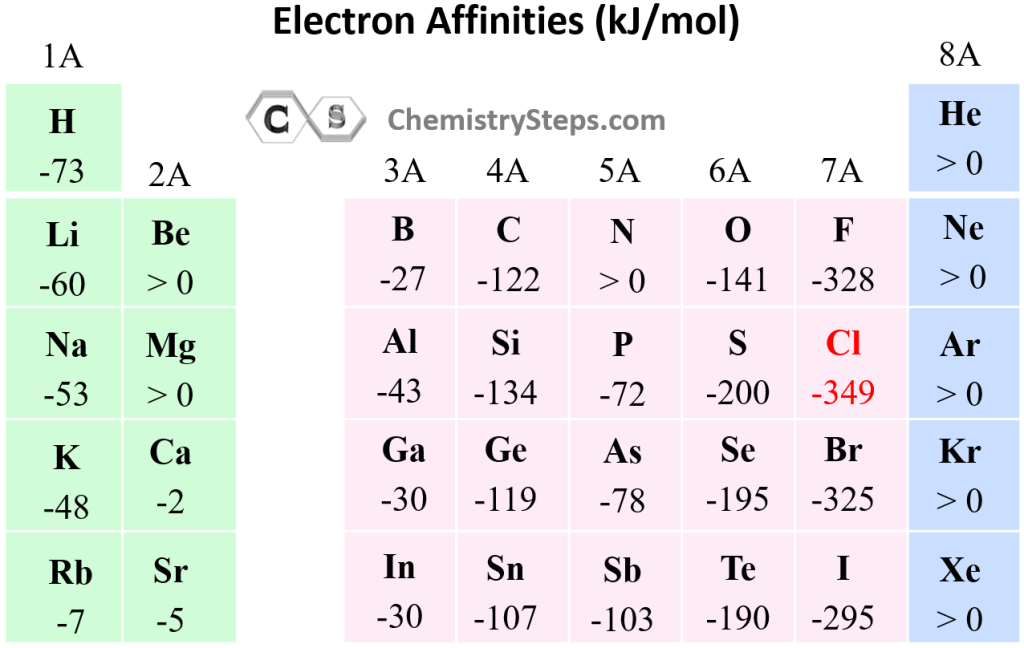
- Across a Period: Generally, electron affinity increases as you move from left to right across a period. This trend arises from the increasing nuclear charge. As the number of protons in the nucleus increases, the attraction between the nucleus and the incoming electron strengthens, leading to a more negative electron affinity. This means the atom releases more energy when it gains an electron.
- Down a Group: Electron affinity generally decreases as you move down a group. This trend is attributed to the increasing distance between the nucleus and the incoming electron. As the electron shells expand, the attraction between the nucleus and the added electron weakens, resulting in a less negative electron affinity.
Factors Affecting Electron Affinity
- Nuclear Charge: As mentioned earlier, a higher nuclear charge leads to a stronger attraction for electrons, resulting in a more negative electron affinity.
- Electron Shielding: Electrons in inner shells shield the outer electrons from the full nuclear charge. This shielding effect weakens the attraction for incoming electrons, leading to a less negative electron affinity.
- Electron Configuration: Atoms with half-filled or completely filled subshells have greater stability. Adding an electron to these configurations disrupts this stability, resulting in a less negative electron affinity.
- Size of the Atom: Smaller atoms generally have higher electron affinities due to a stronger attraction between the nucleus and the incoming electron.
Exceptions to the Trends
While the general trends hold true, there are exceptions to the rules. Some elements exhibit unexpected electron affinity values due to factors like electron configuration, electron-electron repulsion, and the presence of unpaired electrons. For example, nitrogen, with a half-filled p subshell, has a lower electron affinity than oxygen, even though it is located to the left of oxygen in the same period.
Importance of Electron Affinity
Understanding electron affinity is critical for several reasons:
- Predicting Chemical Reactivity: Elements with high electron affinities readily gain electrons, making them strong oxidizing agents. Conversely, elements with low electron affinities tend to lose electrons, acting as reducing agents.
- Bond Formation: The ability of an atom to gain an electron plays a crucial role in the formation of ionic and covalent bonds.
- Understanding Chemical Reactions: Electron affinity helps explain the relative stability of different chemical compounds and predicts the energy changes associated with chemical reactions.
Related Searches:
- Electron Affinity vs. Ionization Energy: These two concepts are closely related. Ionization energy is the energy required to remove an electron from an atom, while electron affinity is the energy released when an atom gains an electron. The two concepts are often used together to understand the overall energy changes involved in chemical reactions.
- Electron Affinity and Electronegativity: Electronegativity is a measure of an atom’s ability to attract electrons within a bond. While both electronegativity and electron affinity reflect an atom’s tendency to gain electrons, electronegativity is a relative concept, comparing the attraction of electrons between two atoms within a bond, while electron affinity is an absolute measure of the energy change when an atom gains an electron.
- Electron Affinity and Chemical Bonding: Electron affinity plays a crucial role in determining the type of chemical bond that forms between atoms. Elements with high electron affinities tend to form ionic bonds, while elements with lower electron affinities tend to form covalent bonds.
- Electron Affinity and Periodic Trends: The trends in electron affinity across a period and down a group can be explained by the interplay of factors like nuclear charge, electron shielding, and electron configuration.
- Electron Affinity and Reactivity: Elements with high electron affinities are often highly reactive as they readily gain electrons to achieve a stable configuration. This reactivity makes them strong oxidizing agents.
- Electron Affinity and Oxidation-Reduction Reactions: Electron affinity is a fundamental concept in understanding oxidation-reduction reactions. Elements with high electron affinities tend to be reduced, gaining electrons, while elements with low electron affinities tend to be oxidized, losing electrons.
- Electron Affinity and Chemical Properties: The electron affinity of an element significantly influences its chemical properties, including its reactivity, the types of bonds it forms, and its ability to participate in various chemical reactions.
- Electron Affinity and Spectroscopy: Spectroscopic techniques can be used to experimentally determine the electron affinity of an element.
FAQs:
- What is the difference between electron affinity and electronegativity? While both concepts relate to an atom’s tendency to attract electrons, electron affinity is an absolute measure of the energy change when an atom gains an electron, while electronegativity is a relative measure of an atom’s ability to attract electrons within a bond.
- How can I predict the electron affinity of an element? While general trends exist, predicting the exact electron affinity of an element requires a deeper understanding of its electronic configuration, nuclear charge, and shielding effects.
- Why is electron affinity important in chemistry? Electron affinity plays a crucial role in predicting chemical reactivity, understanding bond formation, and explaining the stability of chemical compounds.
- Are there any exceptions to the trends of electron affinity? Yes, there are exceptions to the general trends. For example, nitrogen has a lower electron affinity than oxygen, even though it is located to the left of oxygen in the same period. These exceptions arise from factors like electron configuration, electron-electron repulsion, and the presence of unpaired electrons.
Tips:
- Visualize the Periodic Table: Use the periodic table as a visual tool to understand the trends of electron affinity.
- Consider Electron Configuration: The electron configuration of an element plays a significant role in determining its electron affinity.
- Practice with Examples: Work through examples of different elements to solidify your understanding of the trends and exceptions.
- Relate to Other Properties: Connect the concept of electron affinity to other periodic trends like ionization energy and electronegativity.
Conclusion:
Understanding electron affinity is essential for comprehending the fundamental principles of chemistry. The periodic trends in electron affinity provide a valuable framework for predicting chemical reactivity, bond formation, and the overall behavior of elements. By studying these trends, we gain insights into the intricate interplay of forces within atoms and molecules, ultimately contributing to our understanding of the chemical world around us.
.PNG)
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)


Closure
Thus, we hope this article has provided valuable insights into Understanding the Trends of Electron Affinity in the Periodic Table. We appreciate your attention to our article. See you in our next article!